GT Metabolic™ Magnetic Anastomosis System
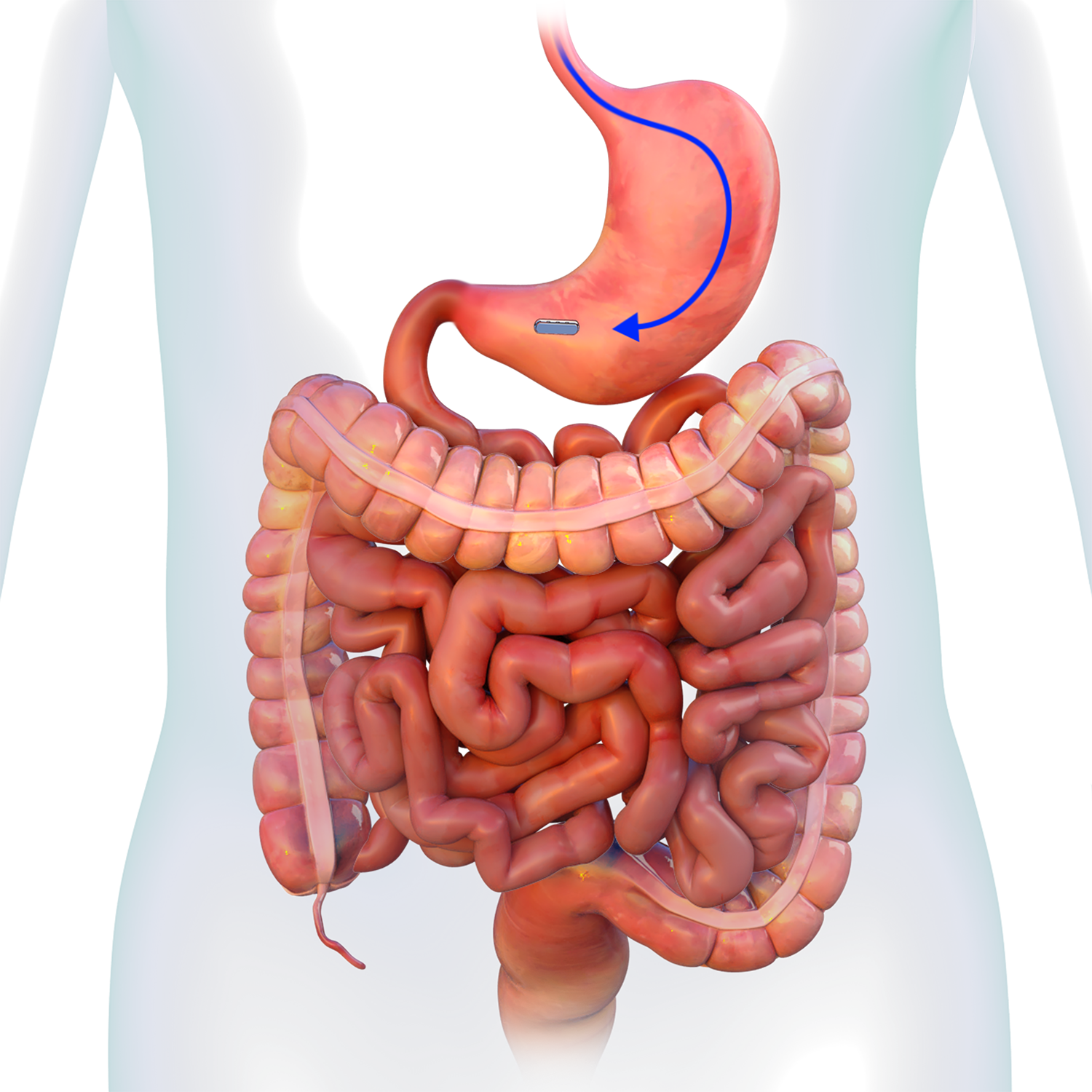
GT Metabolic has developed and is ready to launch a proprietary magnetic compression anastomosis technology.1 The incisionless, sutureless, staple-free technique1-4 is designed to revolutionize how surgeons achieve anastomosis.
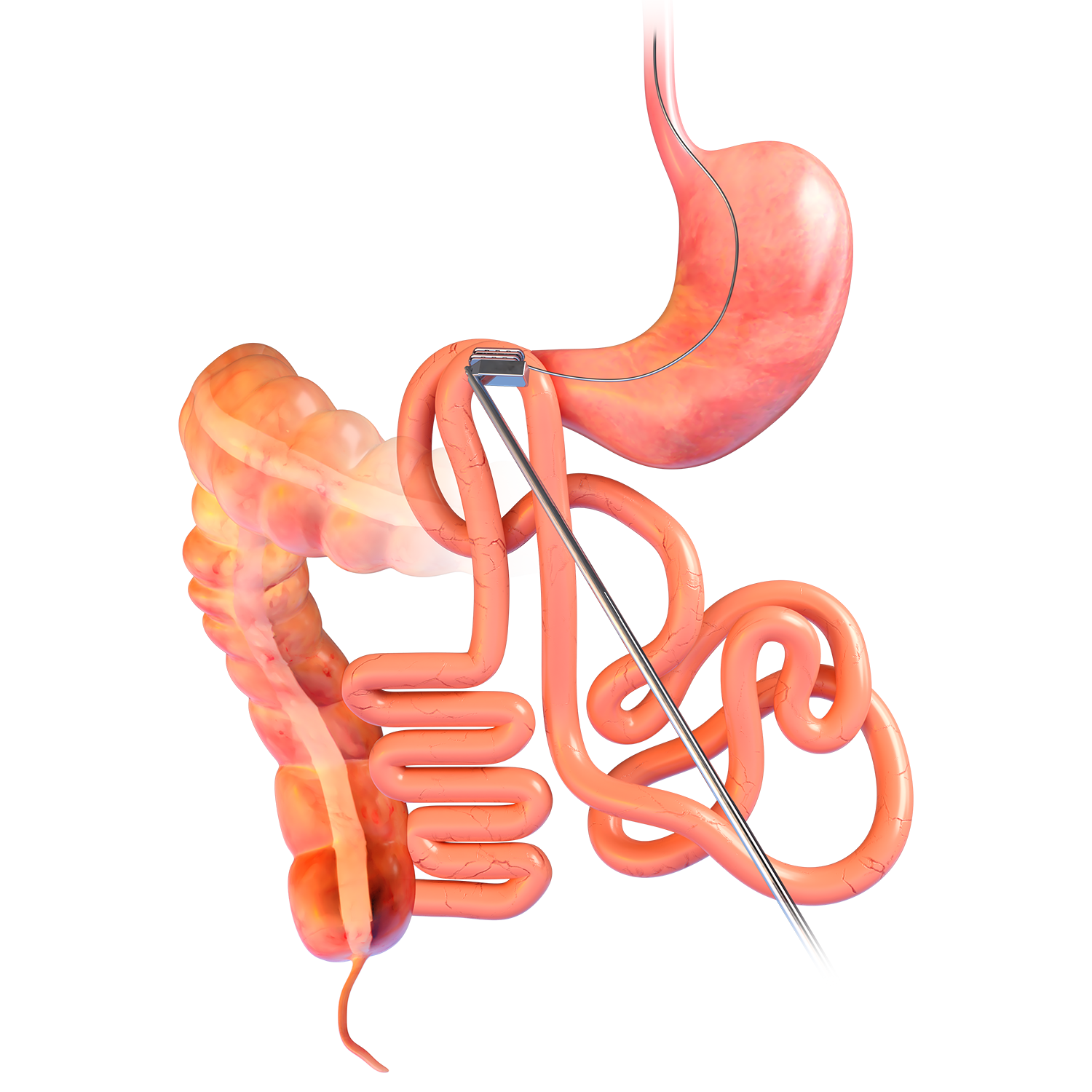
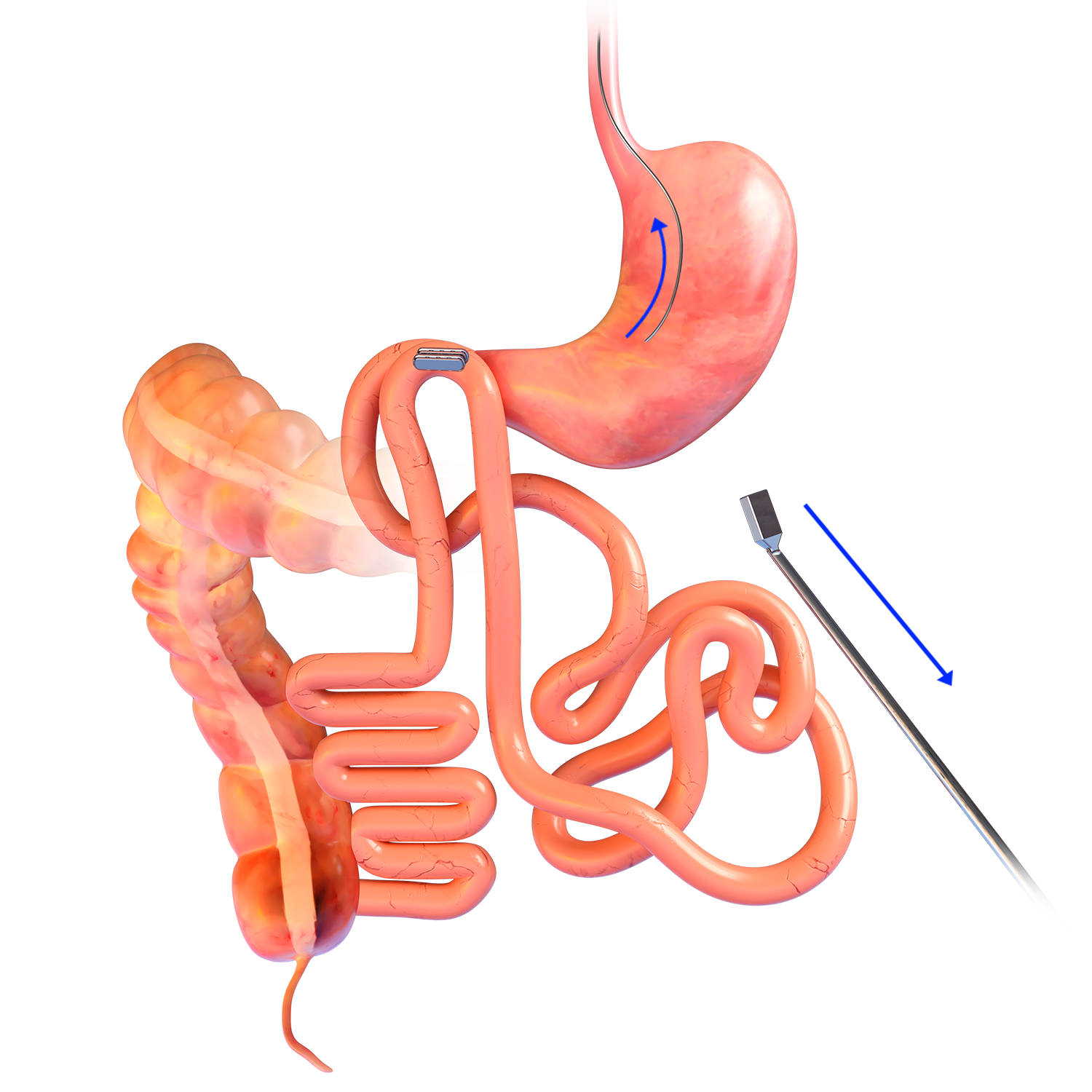
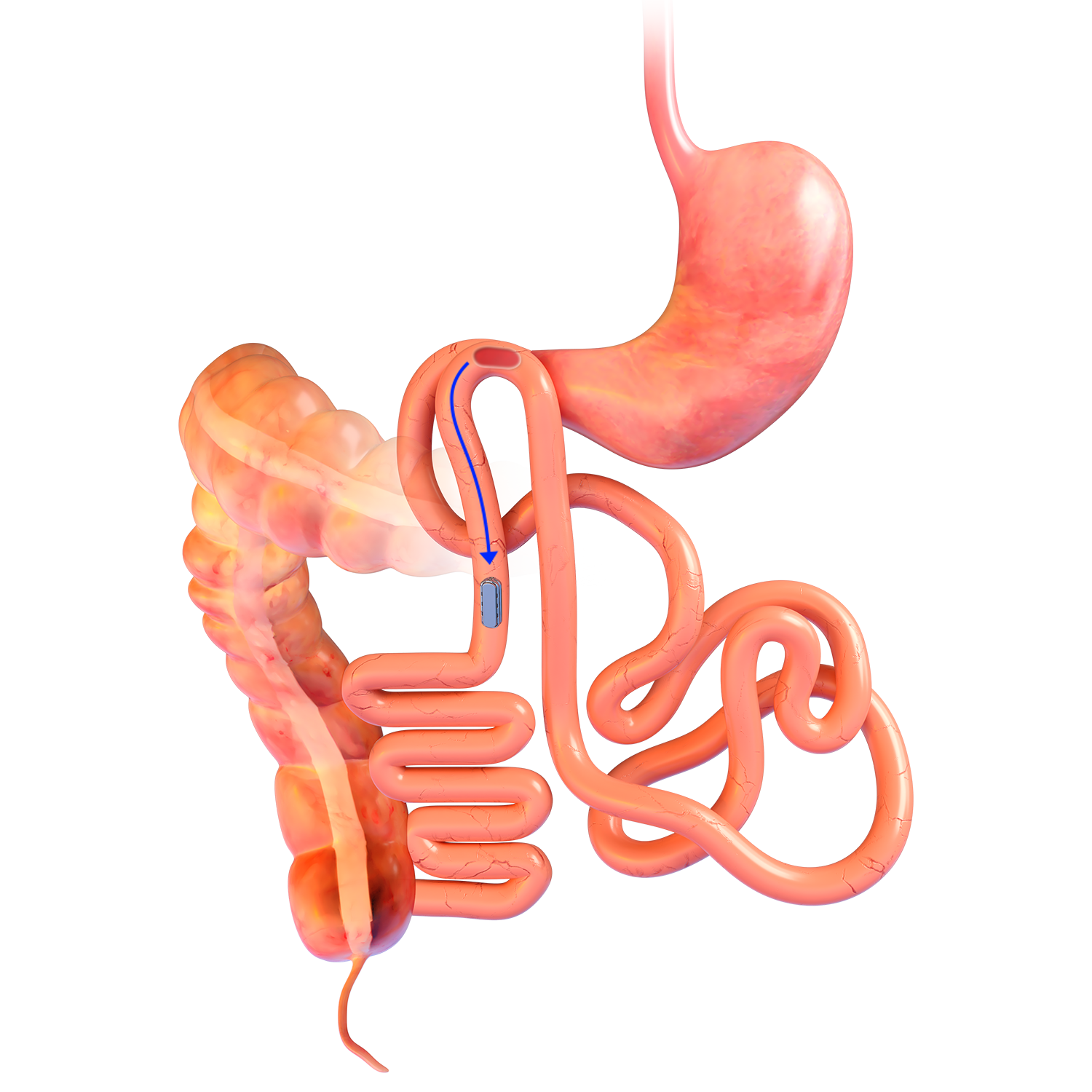
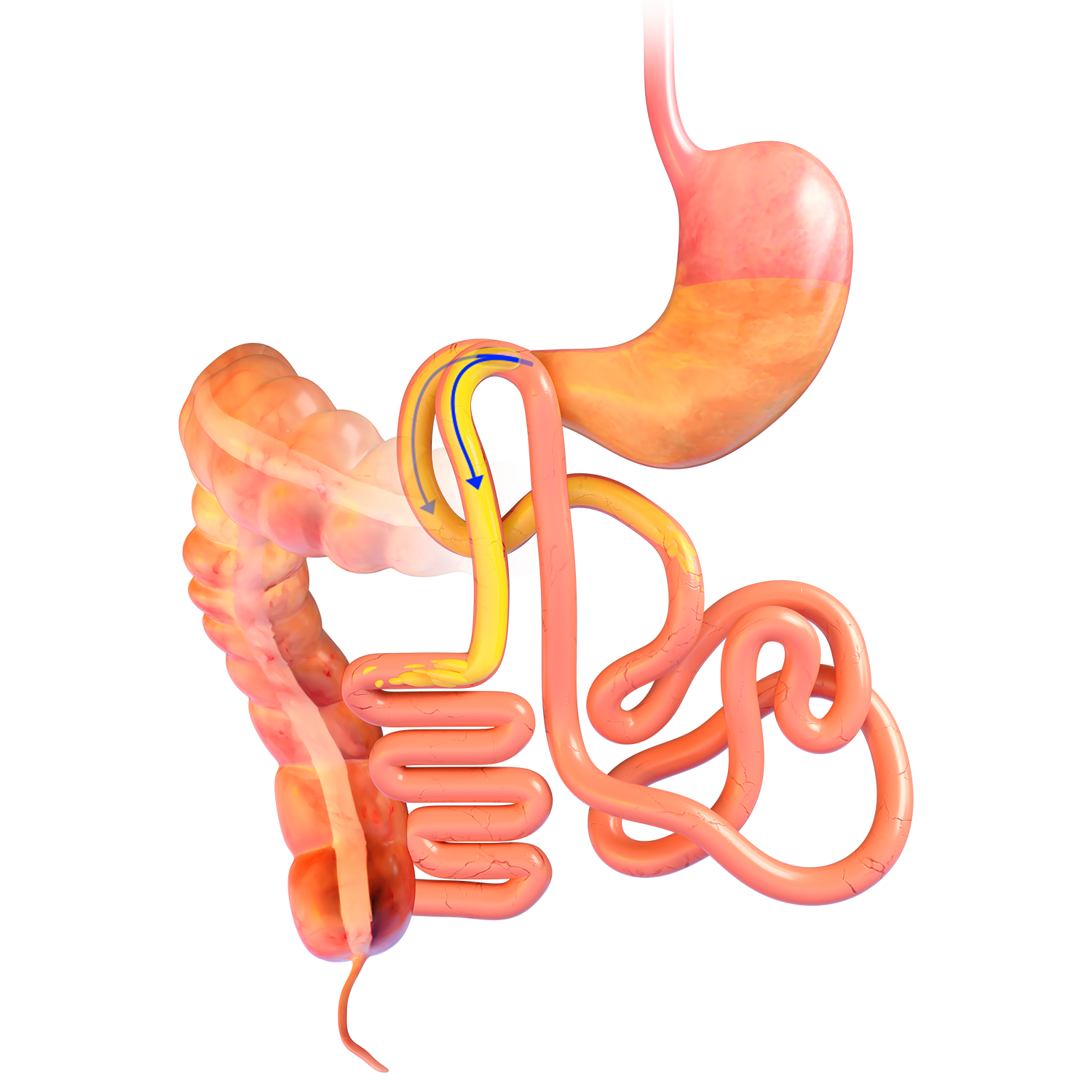
Technique Used In Clinical Studies1-3:
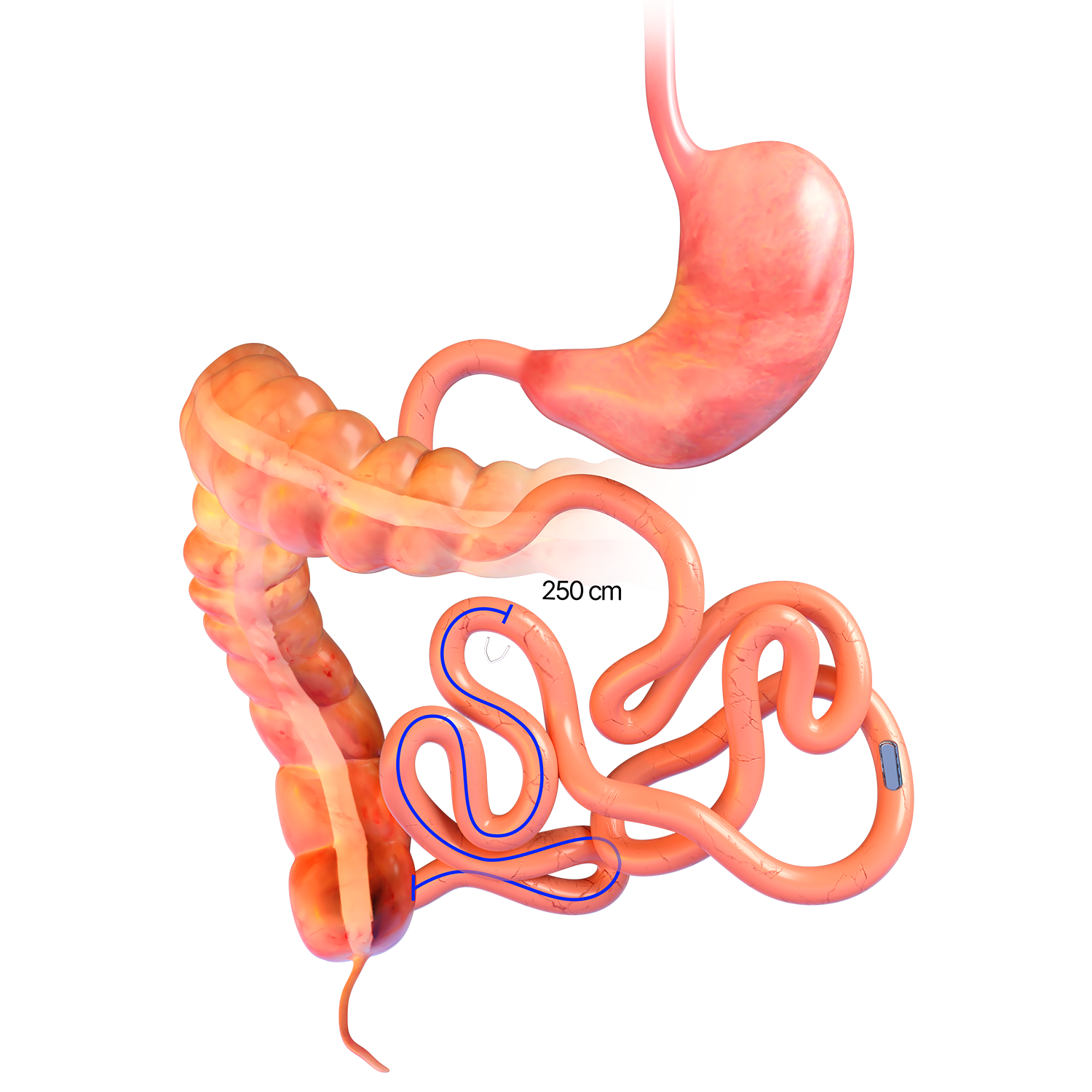
Magnetic System Side-to-Side Compression Anastomosis Duodeno-ileostomy
Surgeon Early Observations
Product Technology
GT Metabolic™ Mag DI™ System
MAGDI™
MAGNETS

- The 40mm1 or 50mm1 Parylene-C coated
neodymium magnet is encased in a titanium shell with a PGLA bio-fragmentable flange. - A delivery system connect/release
mechanism is located at one end of the magnet. - The device is intended for use for side-to-side duodeno-ileal anastomosis in minimally invasive
and laparoscopic surgery.1 - Sterile; disposable.
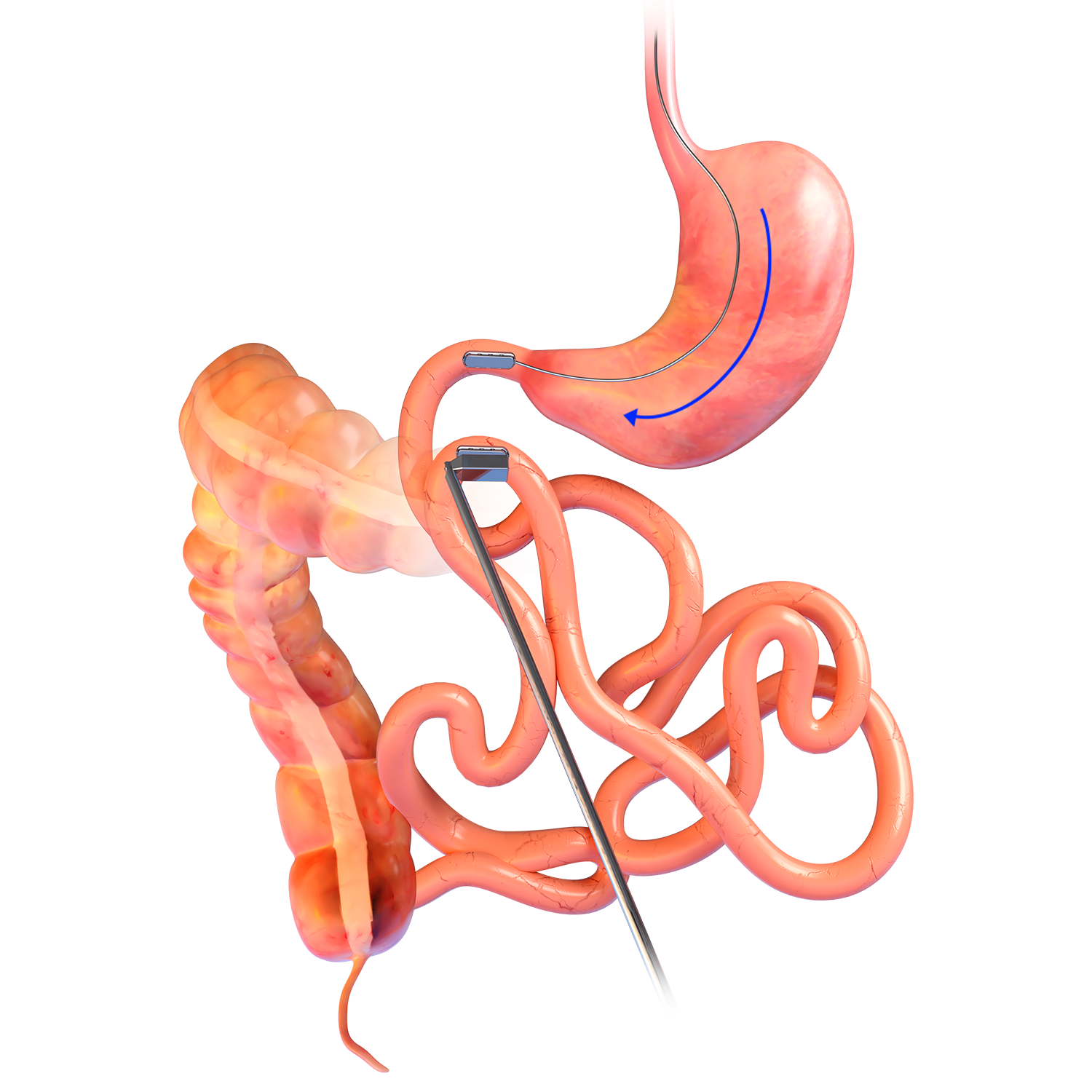
MAGDI™
DELIVERY SYSTEM
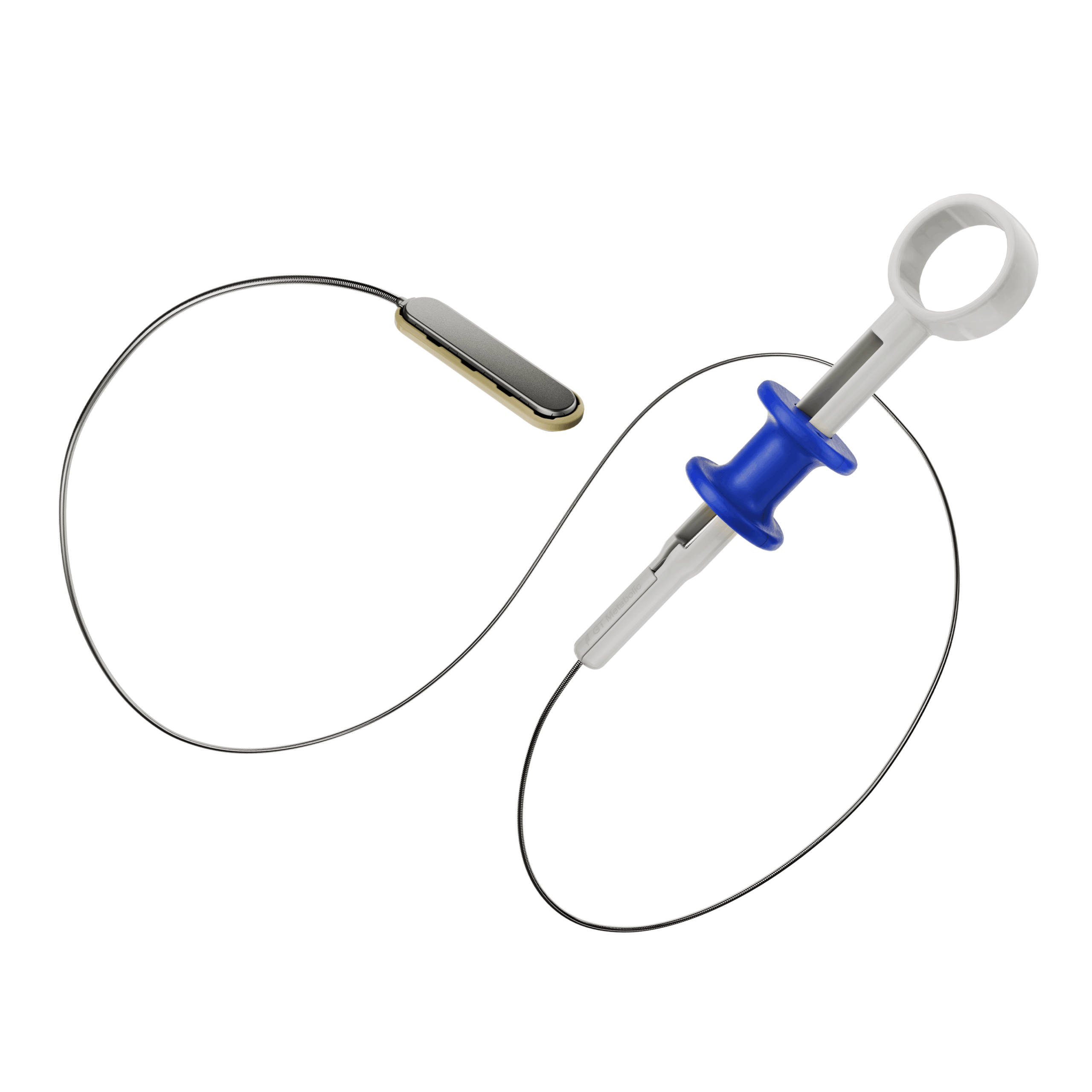
- The delivery system is a disposable, flexible orogastric delivery catheter 198cm in length that attaches to the linear MagDITM magnet for placement and positioning.
- The proximal end of the catheter consists of a ring handle pull trigger for easy deployment of the magnet.
- The delivery system catheter must be paired with a flexible endoscope with a working channel of at least 2.8mm or greater.
- Sterile; disposable.
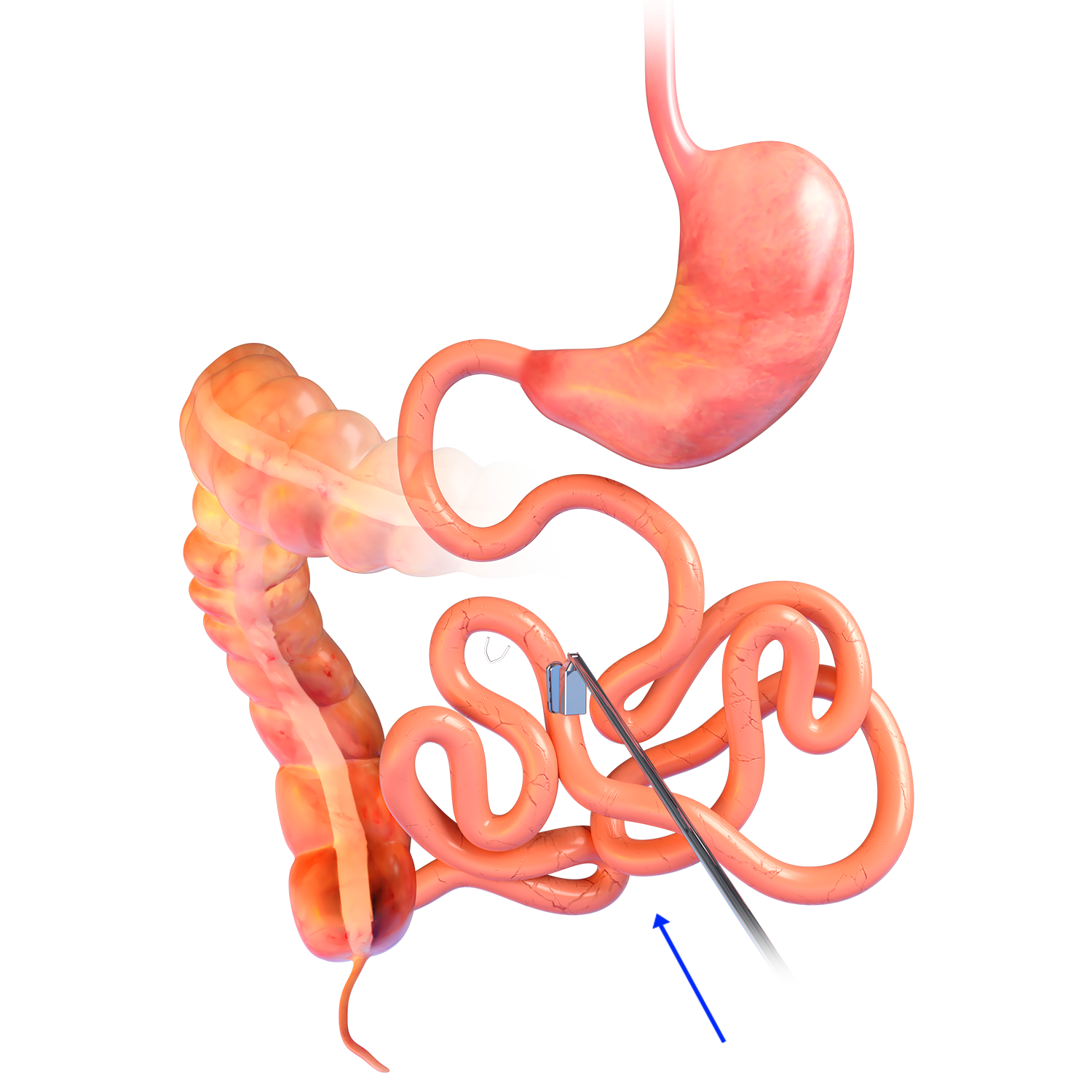
MAGDI™ LAPAROSCOPIC POSITIONING DEVICE

- The 41cm-long reusable laparoscopic
positioning device (LPD) is
compatible with a 12mm trocar. - The instrument is comprised of a Paralene-C coated neodymium magnet encased in a titanium housing, non-magnetic shaft and positioning handle detailing the strength of the instrument.
- Offered in five magnetic strengths, the LPD provides surgeon with options depending on patient’s bowel morphology. (A higher number indicates greater magnetic strength.)
- The LPD is used to position the linear magnetic technology (LMT) at the desired anastomotic site.
- Reusable; requires sterilization.
MAGDI™ MAGNETIC RETRIEVAL DEVICE

- The magnetic retrieval
device (MRD) is a 200cm-long, flexible orogastric
catheter with a magnetic tip - The MRD enables retrieval
of a MagDI™ magnet
if removal is required
after release from the
delivery system during the procedure. - The MRD catheter must
be paired with a flexible
endoscope with a working
channel of at least 2.8mm
or greater. - Sterile; disposable.
The Founders of Our Technology
GT Metabolic founders Thierry Thaure and Michel Gagner, MD, are renowned global experts in the area of bariatric and metabolic procedures. They’ve led the GT Metabolic team in engineering a magnetic compression solution called delayed anastomosis technology (DAT) that surgeons can use to create consistent anastomosis while helping minimize potential complications.1-4
CEO & Co-founder
CMO & Co-founder
2. Gagner, M., Almutlaq, L., Cadiere, G.-B., Torres, A. J., Sanchez-Pernaute, A., Buchwald, J. N., & Abuladze, D. (2023). Side-to-side magnetic duodeno-ileostomy in adults with severe obesity with or without type 2 diabetes: Early outcomes with prior or concurrent sleeve gastrectomy. Surgery for Obesity and Related Diseases. https://doi.org/10.1016/j.soard.2023.10.018
3. Gagner, M., Abuladze, D., Koiava, L. et al.First-in-Human Side-to-Side Magnetic Compression Duodeno-ileostomy with the Magnet Anastomosis System. OBES SURG 33, 2282–2292 (2023). https://doi.org/10.1007/s11695-023-06708-x
4. Gagner M, Cadiere GB, Sanchez-Pernaute A, Abuladze D, Krinke T, Buchwald JN, Van Sante N, Van Gossum M, Dziakova J, Koiava L, Odovic M, Poras M, Almutlaq L, Torres AJ. Side-to-side magnet anastomosis system duodeno-ileostomy with sleeve gastrectomy: early multi-center results. Surg Endosc. 2023 Aug;37(8):6452-6463. doi: 10.1007/s00464-023-10134-6. Epub 2023 May 22. PMID: 37217682; PMCID: PMC10202352.
5. Gagner, M., Abuladze, D., Buchwald, J., et al. First-in-Human Side-to-Side Duodenoileal Bipartition for Weight Loss and Type 2 Diabetes with the Swallowable Biofragmentable Magnetic Anastomosis System. JACS 241(2):p 146-159, August 2025. https://doi.org/10.1097/XCS.0000000000001384.
Want to Follow Our Journey?
Follow Us on LinkedIn
Follow us on LinkedIn to learn more about our publications, poster presentations, conferences, events and product news.
Questions?
We want to hear from you. Please contact us with your questions about our technology, publications, events and other news.